Studying stomatal behavior and transpiration is crucial for understanding how plants respond to environmental stressors like drought and heat. Here are some common techniques used in plant research to measure and analyze stomatal activity and transpiration:
1. Porometry
Porometry is a key technique used in the study of stomatal conductance, which measures how easily gases like water vapor and carbon dioxide move through stomata. Understanding stomatal conductance is crucial because it directly affects important physiological processes like transpiration, the loss of water vapor, and photosynthesis, which involves the uptake of carbon dioxide. Through porometry, researchers can assess the behavior of stomata under various environmental conditions such as drought, changes in light, temperature variations, and humidity levels.
The basic principle of porometry revolves around measuring the diffusion of water vapor from the surface of the leaf into the atmosphere. When stomata are open, water vapor escapes from the leaf, and porometry gauges the rate at which this occurs. The technique provides an understanding of how efficiently stomata are regulating gas exchange and water loss, which are critical for plant survival, especially under stress conditions.
A porometer, the instrument used in this technique, is designed to measure the humidity or vapor pressure near the leaf surface. During the measurement process, a small chamber is clamped onto the leaf, enclosing a section of it. Air is then passed over the leaf inside this chamber. As water vapor diffuses through the stomata, the humidity in the chamber increases. The porometer detects these changes in humidity over time and uses them to calculate the rate of water vapor diffusion. Based on this data, stomatal conductance is determined. Essentially, the porometer calculates how open or closed the stomata are, depending on environmental factors, giving direct insights into the plant’s gas exchange processes.
There are two main types of porometers: steady-state porometers and dynamic porometers. Steady-state porometers take longer for measurements, as they wait for the rate of water vapor loss to stabilize before taking a reading, which makes them very precise. Dynamic porometers, on the other hand, measure the rate of change in water vapor over a set period, making them faster but sometimes less accurate if the environmental conditions are not stable.
Porometry has a wide range of applications, particularly in the study of how stomata respond to environmental stress. Researchers can use it to examine how plants regulate water loss under drought, high temperatures, or changing light conditions. This helps to understand the mechanisms by which plants adjust their water use and gas exchange when under stress. Additionally, porometry is instrumental in determining a plant’s water use efficiency (WUE), as it helps gauge how effectively a plant conserves water while maintaining high stomatal conductance for photosynthesis. The technique is also useful in breeding programs aimed at developing drought-resistant crop varieties, as it allows for the screening of plants that maintain low stomatal conductance under water-limited conditions, indicating better water conservation.
The advantages of porometry include its non-destructive nature, allowing repeated measurements on the same plant over time without causing any harm. Modern porometers are portable, easy to use, and provide real-time data, making them particularly valuable for field studies. They allow researchers to observe immediate plant responses to environmental changes, offering precise insights into how plants manage gas exchange and water loss.
However, porometry has its limitations. It measures stomatal conductance over a small area of the leaf, so the results may not represent the entire plant. Environmental conditions, such as wind or sudden temperature fluctuations, can also affect the accuracy of the readings, making careful control of external factors important during the measurement process.
Despite these limitations, porometry remains an essential tool in plant physiology research, providing critical data on how plants manage their water resources and respond to environmental stresses. It has become a cornerstone technique for understanding the physiological responses of plants to their changing environments, particularly in the context of water use and gas exchange.
2. Infrared Gas Analysis (IRGA)
Infrared Gas Analysis (IRGA) is a sophisticated technique used to study gas exchange in plants, particularly focusing on the rates of photosynthesis and transpiration by measuring changes in gas concentrations, such as carbon dioxide (CO₂) and water vapor. The IRGA technique is based on the principle that different gases absorb specific wavelengths of infrared (IR) light. By measuring how much infrared light is absorbed by a gas, IRGA can determine its concentration in a controlled environment, providing valuable insights into the plant’s physiological activities.
At the core of the IRGA system is the detection of CO₂ and water vapor concentrations in the air surrounding a leaf. Plants take in CO₂ during photosynthesis and release water vapor through transpiration, both of which occur through the stomata. By measuring changes in CO₂ and water vapor levels, IRGA allows researchers to calculate the rate at which plants are photosynthesizing and losing water. This gives a direct measure of plant productivity and water use efficiency, which are essential for understanding how plants respond to varying environmental conditions such as light intensity, temperature, humidity, and drought stress.
The measurement process in IRGA typically involves a leaf being enclosed in a chamber, referred to as a cuvette. Air is passed through this cuvette, and sensors measure the concentration of gases entering and leaving the chamber. As the plant undergoes gas exchange, it absorbs CO₂ and releases water vapor. The IRGA system monitors these changes by directing infrared light through the air sample. Different gases, like CO₂, absorb IR light at specific wavelengths, and the IRGA measures how much light is absorbed. The difference in IR absorption before and after passing through the chamber indicates how much CO₂ has been taken up by the leaf and how much water vapor has been released.
IRGA is widely used to study photosynthesis because it directly quantifies the amount of CO₂ that a plant absorbs for converting light energy into chemical energy. By calculating the CO₂ uptake rate, researchers can determine the photosynthetic efficiency of a plant under different environmental conditions. Transpiration rates can also be measured through IRGA by tracking changes in water vapor concentration in the leaf chamber. This provides a comprehensive view of both water loss and carbon uptake, which are closely linked processes in plant physiology.
IRGA systems come in two primary types: open and closed. In open systems, air continuously flows through the cuvette, and the gas concentrations are measured as air enters and exits the leaf chamber. This allows for a steady-state measurement of gas exchange under natural or controlled environmental conditions. In closed systems, air is sealed within the cuvette, and the concentration of gases is measured over time as they change due to the plant’s gas exchange activities. The closed system allows for precise measurement of gas exchange over short time intervals but may not be as reflective of long-term, steady-state gas exchange as the open system.
The applications of IRGA in plant research are diverse. It is particularly useful in understanding how environmental factors like light, temperature, and water availability affect plant productivity and water use. For example, researchers use IRGA to study how drought stress impacts a plant’s ability to maintain photosynthesis while minimizing water loss. This is crucial for developing drought-resistant crops, as it allows researchers to screen for varieties that can maintain high photosynthetic rates with minimal water use. IRGA is also used in studies related to climate change, helping to assess how plants respond to elevated CO₂ levels and increased temperatures.
One of the main advantages of IRGA is its ability to provide real-time, highly accurate data on gas exchange. This makes it possible to monitor rapid changes in a plant’s physiology in response to environmental stimuli, giving researchers detailed insights into how plants dynamically adjust their photosynthesis and transpiration rates. Additionally, IRGA is non-destructive, allowing repeated measurements on the same plant without causing damage, making it suitable for long-term experiments.
However, IRGA also has its limitations. The equipment is relatively complex and requires precise calibration to ensure accurate measurements. The measurements can also be affected by external factors like temperature and humidity, which need to be carefully controlled during experiments. Additionally, IRGA typically focuses on a small section of the leaf, similar to porometry, which means the results may not always represent the gas exchange behavior of the entire plant.
3. Thermal Imaging (Infrared Thermography)
Thermal imaging, also known as infrared thermography, is a non-invasive technique used to study plant physiology, particularly to monitor temperature changes on the surface of leaves, which provides insights into processes like transpiration, water stress, and heat stress responses. Thermal imaging works by detecting infrared radiation (heat) emitted from an object, in this case, plant leaves, and converting it into a thermal image that represents temperature variations. Plants constantly lose water through transpiration, and this water loss has a cooling effect on leaf surfaces. By using thermal imaging, researchers can measure this cooling effect to assess stomatal behavior and how well plants are managing their water use and heat dissipation under various environmental conditions.
The principle behind thermal imaging is that all objects, including plants, emit infrared radiation as a function of their temperature. The thermal camera detects this radiation and translates it into a color-coded image where different temperatures are represented by varying colors. Cooler areas appear as darker colors, while warmer areas are shown in brighter colors. In plant studies, cooler leaf surfaces typically indicate higher transpiration rates because the evaporation of water from the stomata cools the leaf. Conversely, warmer leaf surfaces may suggest reduced transpiration, which could be due to stomatal closure under stress conditions like drought or heat.
In plant physiology, thermal imaging is particularly useful for monitoring water status. When plants experience water stress, their stomata tend to close to conserve water, reducing transpiration. This results in less evaporative cooling, causing the leaf surface to become warmer. By comparing the thermal images of plants under well-watered and drought conditions, researchers can visually detect water stress by observing temperature differences on the leaf surface. This makes thermal imaging a powerful tool for assessing drought tolerance in plants. For example, plants that maintain cooler leaf temperatures under water-limited conditions are generally more efficient at managing water use and may have mechanisms that enable them to continue transpiration at optimal rates even when water is scarce.
Thermal imaging also plays a significant role in studying heat stress. When plants are exposed to high temperatures, they face increased risks of thermal damage, and their cooling mechanism—transpiration—becomes crucial. Thermal cameras can track how effectively plants are dissipating heat under these conditions. If a plant cannot maintain adequate transpiration due to stomatal closure or environmental limitations, its leaf surface temperature will rise. Thermal imaging helps researchers determine how different plant species or cultivars handle heat stress and identifies those that can maintain cooler leaf temperatures, which is an important trait for heat tolerance.
In addition to its applications in water and heat stress studies, thermal imaging is used to investigate stomatal behavior. By capturing temperature changes over time, it is possible to observe dynamic stomatal responses to environmental stimuli such as light, humidity, or the application of abscisic acid (ABA), a hormone that induces stomatal closure during drought. Thermal imaging allows researchers to map spatial variations in stomatal activity across the leaf surface, providing a detailed understanding of how stomata respond to localized environmental conditions.
One of the major advantages of thermal imaging is that it is a non-contact and non-destructive technique. Researchers can monitor entire plants or canopies in real time without disturbing the plants or altering their natural behavior. This is particularly useful for field studies where plants are exposed to natural environmental conditions. Thermal imaging can also be applied across different scales, from individual leaves to whole plant canopies, allowing researchers to assess the water and heat status of large plant populations in agricultural fields.
However, the accuracy of thermal imaging depends on certain environmental factors. For instance, external sources of heat, such as direct sunlight, can affect the accuracy of the temperature readings, as can wind or fluctuating humidity levels. Therefore, careful calibration and control of environmental variables are necessary for precise measurements. Additionally, while thermal imaging provides an indirect measure of stomatal conductance and water stress, it does not directly measure gas exchange, so it is often used in combination with other techniques, such as porometry or infrared gas analysis (IRGA), for a more comprehensive understanding of plant physiological responses.
4. Chlorophyll Fluorescence
Chlorophyll fluorescence is a widely used technique in plant physiology to assess the health and efficiency of the photosynthetic process. It involves the measurement of light re-emitted by chlorophyll molecules during photosynthesis, offering insights into how well plants are converting light energy into chemical energy. Chlorophyll fluorescence is particularly valuable because it provides a non-invasive, real-time method for monitoring the activity of Photosystem II (PSII), the part of the photosynthetic machinery responsible for the initial steps of capturing light energy.
The principle behind chlorophyll fluorescence lies in how plants manage absorbed light. When chlorophyll in plant cells absorbs light, three things can happen: the energy can be used for photosynthesis (photochemistry), dissipated as heat, or re-emitted as fluorescence. Chlorophyll fluorescence represents only a small fraction of the absorbed light (usually less than 5%), but by measuring this fluorescence, researchers can infer the efficiency of photochemistry and detect disruptions in the photosynthetic process caused by environmental stress, nutrient deficiencies, or disease.
Chlorophyll fluorescence is typically measured using fluorometers, which emit brief pulses of light onto a leaf or plant sample and then capture the emitted fluorescence. This measurement allows researchers to assess the performance of PSII under various conditions. One of the key parameters obtained from chlorophyll fluorescence is the maximum quantum efficiency of PSII (Fv/Fm). This ratio is an indicator of the potential efficiency of PSII in dark-adapted leaves and reflects the plant’s photosynthetic capacity. Under optimal conditions, the Fv/Fm value for most plants is around 0.83, and deviations from this value indicate stress or damage to the photosynthetic apparatus.
Chlorophyll fluorescence is particularly useful for studying stress responses in plants. For instance, under drought, heat, or excessive light conditions, plants can experience damage to their photosynthetic systems, leading to a reduction in photochemical efficiency. By monitoring changes in fluorescence, researchers can detect early signs of stress before visible symptoms, such as wilting or chlorosis, appear. This makes chlorophyll fluorescence an effective diagnostic tool in agriculture for identifying crops under stress or evaluating the effectiveness of treatments designed to alleviate environmental or nutrient stresses.
In addition to Fv/Fm, there are other parameters used in chlorophyll fluorescence analysis, such as non-photochemical quenching (NPQ), which measures how much of the absorbed light energy is being dissipated as heat. NPQ reflects the plant’s protective mechanisms to avoid damage from excess light, particularly under conditions of stress. When light intensity is too high, plants divert excess energy to protect the photosynthetic apparatus from damage, a process that is captured by changes in NPQ. By monitoring NPQ, researchers can gain insights into how plants are adjusting to high light or other stressors.
Another commonly measured parameter is the electron transport rate (ETR), which provides an estimate of how efficiently electrons are moving through the photosynthetic electron transport chain. This measurement is useful in determining how well plants are performing photosynthesis under different environmental conditions.
Chlorophyll fluorescence is also applied in screening for stress tolerance in plants. Researchers use this technique to evaluate different plant varieties for their ability to maintain high photosynthetic efficiency under stress conditions such as drought, heat, or cold. Plants that show less reduction in Fv/Fm or higher NPQ under stress are generally considered more stress-tolerant. This makes chlorophyll fluorescence an important tool in crop breeding programs aimed at developing more resilient varieties in response to climate change.
One of the major advantages of chlorophyll fluorescence is its non-destructive nature, allowing repeated measurements on the same plant over time without causing harm. This makes it ideal for long-term monitoring of plant health in both field and laboratory settings. Additionally, chlorophyll fluorescence can be measured quickly, providing real-time data on the physiological state of the plant, which is useful for both basic research and practical applications in agriculture.
However, like any technique, chlorophyll fluorescence has its limitations. Environmental factors such as temperature, light intensity, and leaf structure can influence fluorescence readings, so it is essential to carefully control experimental conditions to obtain accurate and reproducible results. Moreover, chlorophyll fluorescence primarily provides information about the efficiency of PSII and does not directly measure the rate of carbon fixation, so it is often used in conjunction with other techniques such as gas exchange analysis to obtain a more comprehensive picture of plant photosynthesis.
5. Pressure Bomb (Pressure Chamber)
The pressure bomb, also known as the pressure chamber, is a widely used tool in plant physiology for measuring water potential, particularly in the leaves and stems of plants. Water potential indicates how tightly water is held within a plant, and it is a key factor in understanding plant water relations, especially under conditions of drought stress. By using the pressure bomb, researchers can gain insights into the plant’s water status, helping to assess its ability to cope with water limitations and its overall hydration.
The principle behind the pressure bomb is relatively simple. When a plant is well-hydrated, water moves easily through its vascular system. But when a plant experiences water stress, the tension in its water columns increases as water is drawn up from the roots to the leaves. The pressure bomb measures this tension by applying external pressure to force water out of a cut leaf or stem, mimicking the internal tension the plant experiences during normal transpiration.
In practice, the technique works as follows: a leaf (or a small stem) is detached from the plant, and the cut end is sealed inside a pressurized chamber, while the leaf blade or part of the stem remains outside. Compressed gas, typically nitrogen, is gradually introduced into the chamber, increasing the pressure. As pressure builds inside the chamber, it pushes water from the xylem (the plant’s water-conducting tissue) toward the cut surface. When the water begins to appear at the cut end of the leaf or stem, the applied pressure is recorded. This pressure corresponds to the tension the water column was under within the plant, and it is directly related to the water potential of the plant tissue.
The pressure bomb allows researchers to measure water potential at various points in the plant, providing valuable information about the plant’s ability to transport water under different environmental conditions. This is especially important during drought stress, where water potential decreases as the plant struggles to maintain adequate water levels. By measuring water potential, researchers can assess the severity of drought stress a plant is experiencing and how well it can cope with reduced water availability.
A key advantage of the pressure bomb technique is its ability to provide direct, quantitative data on the water status of plants. The water potential measurements help researchers understand the plant’s capacity to transport water through its vascular system, a process essential for maintaining turgor pressure, nutrient transport, and photosynthesis. The technique is also widely used in agriculture to monitor crop water stress and inform irrigation practices. By regularly measuring water potential in crops, farmers can determine the optimal timing for irrigation, ensuring that plants receive water before experiencing severe stress, which can reduce yield and quality.
The pressure bomb is particularly valuable for screening drought tolerance in plants. Species or varieties that maintain higher water potential under drought conditions are generally considered more drought-tolerant because they are better able to maintain water transport through the xylem, even as soil moisture declines. In this context, the pressure bomb can help identify plant varieties with better water-use efficiency or greater resilience to dry conditions.
While the pressure bomb is a powerful tool, it does have some limitations. One of the main challenges is that the measurement is destructive—once a leaf or stem is cut, it cannot be used for further measurements, limiting the ability to take repeated measurements on the same tissue. Also, the technique provides a snapshot of the plant’s water status at the moment the sample is taken, but it does not give continuous or long-term data. To overcome this, researchers often take multiple measurements over time and from different parts of the plant to build a more comprehensive picture of its water relations.
Additionally, the accuracy of the pressure bomb technique can be influenced by factors such as leaf age, environmental conditions, and the species being studied. Therefore, careful experimental design and consistent sampling protocols are necessary to ensure reliable results.
Despite these limitations, the pressure bomb remains one of the most commonly used methods for studying plant water relations due to its ease of use, portability, and ability to provide direct measurements of water potential in the field or laboratory. By understanding a plant’s water potential, researchers and farmers can make informed decisions about water management, breeding programs, and strategies for improving drought tolerance.
6. Leaf Gas Exchange Measurements
Leaf gas exchange measurements are a fundamental technique in plant physiology used to study the rates of photosynthesis, transpiration, and stomatal conductance in plants. These measurements provide direct insights into how plants regulate gas exchange—particularly the uptake of carbon dioxide (CO₂) for photosynthesis and the release of water vapor through transpiration. By analyzing gas exchange, researchers can understand plant responses to environmental conditions such as light, temperature, humidity, and water availability, making it a critical tool for studying plant productivity and stress tolerance.
The process of leaf gas exchange is primarily regulated by stomata, small openings on the surface of leaves that control the flow of gases into and out of the plant. When stomata open, CO₂ enters the leaf to be used in photosynthesis, while water vapor is simultaneously lost to the atmosphere through transpiration. By measuring the exchange of these gases, researchers can assess the efficiency of photosynthesis (the plant’s ability to convert light energy into chemical energy) and the plant’s water-use efficiency (the ratio of carbon gained to water lost).
Gas exchange measurements are typically conducted using infrared gas analyzers (IRGAs) or other gas-exchange systems. These instruments measure the concentration of CO₂ and water vapor in air passing over a leaf, allowing researchers to calculate the rates of photosynthesis, transpiration, and stomatal conductance. The basic principle involves enclosing a leaf in a cuvette, or small chamber, where controlled air is passed over the leaf. The concentration of gases is measured both before and after the air passes over the leaf, providing data on how much CO₂ the leaf has absorbed and how much water vapor it has released.
There are several key parameters that can be derived from leaf gas exchange measurements:
- Photosynthetic rate (A): This is the rate at which a leaf is assimilating CO₂ for photosynthesis. It is typically measured in micromoles of CO₂ per square meter per second (µmol CO₂ m⁻² s⁻¹). The photosynthetic rate is influenced by factors such as light intensity, CO₂ concentration, temperature, and the availability of water and nutrients.
- Transpiration rate (E): This refers to the rate at which water vapor is lost from the leaf to the atmosphere. Transpiration is driven by the vapor pressure difference between the inside of the leaf and the surrounding air, and it is measured in millimoles of water per square meter per second (mmol H₂O m⁻² s⁻¹). Transpiration plays a key role in regulating leaf temperature and transporting nutrients within the plant.
- Stomatal conductance (gs): This is a measure of how easily gases (CO₂ and water vapor) move through the stomata. It reflects the degree of stomatal opening, which controls both the rate of CO₂ uptake for photosynthesis and water loss through transpiration. Stomatal conductance is measured in mol H₂O m⁻² s⁻¹, and it is a crucial parameter for understanding how plants balance their need for CO₂ with their need to conserve water.
- Intercellular CO₂ concentration (Ci): This is the concentration of CO₂ inside the leaf, specifically within the air spaces between mesophyll cells where photosynthesis takes place. Ci provides information about the efficiency of CO₂ use in the Calvin cycle, the biochemical pathway through which plants fix carbon. When Ci is low, it suggests that CO₂ is being efficiently used in photosynthesis. When Ci is high, it may indicate that photosynthesis is limited by other factors, such as insufficient light or enzyme activity.
- Water-use efficiency (WUE): This is the ratio of photosynthesis to transpiration, often expressed as µmol CO₂ mmol⁻¹ H₂O. Water-use efficiency is an important indicator of how well a plant is using water to assimilate carbon. High WUE suggests that a plant is effectively balancing CO₂ uptake with minimal water loss, which is critical in water-limited environments such as during drought conditions.
Leaf gas exchange measurements are highly sensitive to environmental variables, making them useful for studying how plants respond to changing conditions. For example, drought stress typically leads to stomatal closure, which reduces both transpiration and CO₂ uptake, ultimately limiting photosynthesis. By comparing gas exchange parameters under well-watered and drought conditions, researchers can assess a plant’s drought tolerance and its ability to maintain photosynthetic efficiency while conserving water.
Similarly, gas exchange measurements are essential for understanding how plants respond to heat stress. Under high temperatures, plants may increase transpiration to cool their leaves, but this also leads to greater water loss. Gas exchange data can help determine the threshold at which stomatal regulation breaks down under heat stress, leading to reduced photosynthesis and increased susceptibility to water loss.
Leaf gas exchange is also used to study the effects of elevated CO₂ levels, as part of research on climate change. Plants exposed to higher atmospheric CO₂ concentrations often show increased photosynthesis and water-use efficiency, but the long-term effects on growth, resource allocation, and crop yield are still being investigated. Gas exchange measurements help researchers understand how different plant species or genotypes may respond to future environmental conditions.
One of the major advantages of leaf gas exchange measurements is their ability to provide real-time, dynamic data on photosynthetic performance and water use. This allows researchers to observe rapid changes in plant physiology in response to environmental fluctuations, such as a sudden drop in humidity or an increase in temperature. The non-destructive nature of the technique means that multiple measurements can be taken over time on the same plant, providing detailed information on how gas exchange processes change throughout the day or growing season.
However, gas exchange measurements are typically limited to individual leaves or small areas of a plant, so they may not always reflect the behavior of the entire plant or canopy. To address this limitation, researchers often complement leaf gas exchange measurements with other techniques, such as chlorophyll fluorescence or thermal imaging, to gain a more complete picture of the plant’s physiological status.
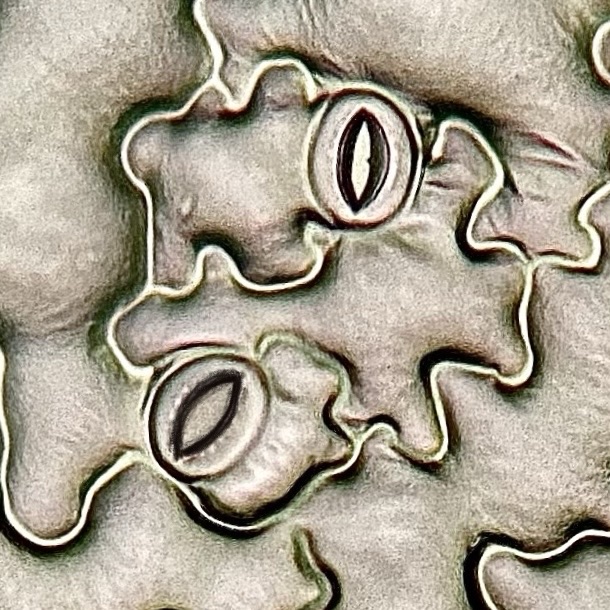
7. Microscopy (Light and Scanning Electron Microscopy)
Microscopy, specifically light microscopy (LM) and scanning electron microscopy (SEM), are vital tools in plant science for examining the intricate structures of plant tissues, including stomata, cell walls, and vascular tissues. Both techniques allow researchers to observe plant anatomy and morphology at different levels of detail, helping to understand plant development, physiology, and responses to environmental stresses such as heat and drought.
Light Microscopy (LM)
Light microscopy uses visible light to illuminate samples, making it one of the most common techniques for examining plant tissues. With light microscopy, plant cells and tissues can be observed at magnifications typically ranging from 40x to 1000x, providing detailed views of structures such as stomata, leaf epidermis, xylem, and phloem. Light microscopes are widely accessible and come in various types, including bright-field, phase-contrast, and fluorescence microscopes, each serving different purposes depending on the research goals.
In bright-field microscopy, light passes through the plant sample, and the contrast between different structures is created by staining the tissues with dyes, making the cells or specific organelles more visible. Commonly used stains in plant microscopy include safranin, which binds to lignin, and iodine, which stains starch granules. This method is particularly useful for studying general plant anatomy, such as the arrangement of stomata on the leaf surface, the thickness of the cell walls, or the structure of the vascular system.
Phase-contrast microscopy allows the observation of living plant cells without the need for staining. It enhances the contrast of transparent samples, making it ideal for studying dynamic processes like stomatal movement or cytoplasmic streaming in live cells.
Fluorescence microscopy, another variation of light microscopy, uses fluorescent dyes or proteins to label specific structures within the plant. This technique is often employed in plant physiology to study cellular components like chloroplasts, mitochondria, and nuclei or to track the movement of proteins within cells. For instance, scientists may use fluorescence microscopy to visualize stomatal behavior by labeling guard cells with a fluorescent marker, helping them understand how stomata open and close in response to environmental signals.
Light microscopy is particularly effective for observing stomatal density and distribution. By examining leaf peels or cross-sections of leaves under a light microscope, researchers can measure the number and distribution of stomata per unit area. This is essential for studying how different plants adapt to drought or heat stress. Plants with fewer stomata or those that regulate stomatal aperture more tightly tend to lose less water through transpiration, making them more drought-tolerant.
Scanning Electron Microscopy (SEM)
While light microscopy provides a good view of cellular structures, scanning electron microscopy offers much higher magnifications (ranging from 20x to over 100,000x) and greater resolution, enabling the detailed visualization of surface structures in three dimensions. SEM is particularly useful for examining the ultra-structure of plant tissues, such as the detailed morphology of stomata, trichomes, and cell walls, and for understanding the finer aspects of plant responses to environmental stresses.
In SEM, a beam of electrons is scanned across the surface of the plant sample, creating detailed images by detecting the electrons that bounce off the sample. Because SEM uses electrons rather than light, it can resolve structures at the nanometer scale, providing much higher resolution than light microscopy. The images produced by SEM are highly detailed and show the surface topography of plant tissues in three dimensions, making it an excellent tool for studying surface features like stomatal pores, epidermal cell patterns, and cuticle structure.
Before SEM analysis, plant samples must be prepared through a process called fixation, which preserves their structure. The samples are typically dehydrated and coated with a thin layer of conductive material, such as gold or platinum, to prevent the buildup of electrical charge when exposed to the electron beam. This preparation process ensures that the fine details of the plant surface are maintained and can be visualized with high fidelity.
SEM is particularly valuable for studying stomatal morphology under stress conditions, such as drought or heat. By using SEM, researchers can observe changes in the size, shape, and aperture of stomata in response to environmental changes. For example, during drought stress, SEM can reveal how stomata shrink or become more closed to reduce water loss through transpiration. Similarly, the technique can be used to study the deposition of waxes or other protective substances on the leaf surface, which plants may produce to reduce water loss under extreme heat or dry conditions.
In addition to stomata, SEM is also used to examine other surface structures, such as trichomes (hair-like structures on plant leaves and stems), which play roles in protecting plants from herbivores, reducing water loss, or reflecting excess light. The detailed images provided by SEM help researchers understand the role of these structures in plant survival under different environmental conditions.
One of the most significant advantages of SEM over light microscopy is its ability to produce highly detailed, three-dimensional images of plant surfaces, allowing for a better understanding of the spatial relationships between structures. This makes SEM an excellent tool for investigating the surface anatomy of leaves, stems, and seeds.
Both light microscopy and SEM are frequently used to study plant responses to environmental stresses like drought and heat. For instance, under drought conditions, researchers can use light microscopy to examine changes in the structure of vascular tissues, such as xylem, to understand how plants adjust their water transport systems. Similarly, SEM can be used to observe the deposition of epicuticular waxes, which form a barrier against water loss and help plants survive in arid environments.
The two techniques also complement each other well. Light microscopy is ideal for studying internal structures and processes, while SEM is unparalleled for examining surface features at high resolution. By combining both methods, researchers can gain a comprehensive understanding of how plants respond to stress at both the cellular and tissue levels.
8. Sap Flow Sensors
Sap flow sensors are crucial tools in plant physiology, used to measure the movement of water within a plant’s vascular system, primarily the xylem. The flow of sap, which consists mainly of water and dissolved nutrients, plays an essential role in transporting vital substances from the roots to the leaves, supporting processes like photosynthesis and overall plant metabolism. Measuring sap flow provides insight into the plant’s water consumption, its transpiration rates, and its response to varying environmental conditions such as drought, heat, and humidity.
The importance of sap flow measurements lies in their ability to provide a clear understanding of plant water use, particularly under stress conditions. These measurements help assess how efficiently plants use water, how much water is being lost through transpiration, and how plants adapt to drought or heat stress. The data obtained is especially valuable for improving irrigation practices, optimizing water use in agriculture, and investigating plant water stress resistance.
Sap flow sensors typically function by measuring the rate at which water moves through the plant’s xylem. The most common techniques for measuring sap flow involve heat-based methods, where heat is applied to the plant, and temperature changes due to water movement are tracked. One such method is the Heat Pulse Velocity (HPV) technique, which uses a brief pulse of heat introduced into the stem, followed by temperature measurements from sensors placed both above and below the heated section. The speed of the heat’s movement indicates the velocity of sap flow. This method is particularly useful for examining changes in water transport under different conditions, such as during drought or well-watered phases.
Another method, the Heat Balance technique, applies constant heat to a portion of the plant stem and measures how much of this heat is transported away by the sap flow. Sensors placed above and below the heated section help determine the temperature distribution, which is then used to calculate sap flow rates. This method allows continuous monitoring of sap flow and is ideal for studying daily patterns of water use in larger plants or trees, providing valuable information on water use dynamics.
The Thermal Dissipation (Granier) method is another widely used approach for measuring sap flow, particularly in trees and larger plants. In this technique, a heated probe is inserted into the plant’s stem, and the temperature difference between the heated and unheated parts of the stem is measured. The rate at which heat dissipates correlates with the velocity of sap flow. This method is favored for its simplicity and reliability, offering long-term, continuous sap flow data. Researchers often use this method to assess water use in forests or to study how trees respond to water stress or changes in environmental conditions.
Sap flow sensors have a wide range of applications in plant physiology and environmental research. One key application is in agriculture, where sap flow data is used to optimize irrigation practices. By monitoring sap flow in crops, farmers can determine the exact water requirements of their plants and adjust irrigation schedules accordingly. This ensures that crops receive enough water to maintain growth and productivity while avoiding over-irrigation, which can lead to water wastage. Sap flow data is also vital for precision agriculture systems that aim to apply water more efficiently.
In drought stress research, sap flow sensors are used to study how water transport changes as soil moisture levels drop. Plants typically reduce sap flow under drought conditions to conserve water, often closing their stomata to limit transpiration. By monitoring sap flow, researchers can identify drought-tolerant plants that maintain water transport under limited water conditions. This knowledge is particularly important in breeding programs focused on developing crops suited to arid environments.
Sap flow sensors are also valuable tools for studying plant responses to heat stress. Under high temperatures, plants often increase transpiration to cool themselves through the evaporation of water from leaf surfaces. This leads to higher sap flow as more water is transported from the roots to the leaves. However, if heat stress is combined with water scarcity, sap flow may decrease as the plant conserves water. Monitoring these changes helps researchers understand how plants balance cooling mechanisms with water conservation under heat stress.
In forestry and ecological research, sap flow measurements are used to study how trees and forests respond to environmental changes. Sap flow data can help determine which tree species are more resilient to drought or heat and how forest water use patterns change during periods of stress. This information is valuable for managing forest ecosystems, particularly in the context of climate change, where water availability may become less predictable.
Sap flow sensors are also used in fundamental plant physiology research to study how water transport within plants is regulated. The data obtained from sap flow measurements can be linked with other physiological processes, such as stomatal conductance, leaf water potential, and overall plant growth. These insights help researchers develop models that predict plant responses to environmental changes and contribute to better understanding plant-environment interactions.
One of the main advantages of sap flow sensors is their ability to provide continuous, real-time data on plant water use. This allows for long-term monitoring of how water use changes in response to environmental conditions, such as daily shifts in light, humidity, and temperature. Sap flow sensors are relatively non-invasive, making them ideal for long-term studies on individual plants without causing significant damage. This feature is particularly valuable for assessing how the same plants respond to stress over extended periods.
However, sap flow measurement does have limitations. The accuracy of the measurements can be influenced by factors such as the plant’s stem size, wood density, and the precise placement of the sensors. Additionally, sap flow sensors typically measure water movement in the xylem but do not provide information on other processes like water uptake from the roots or water loss through the leaves, which must be studied using complementary methods.
Another challenge is that sap flow sensors are generally more suited to larger plants or trees due to the need for sufficient stem diameter to insert the probes. For smaller plants, other methods, such as gravimetric techniques or leaf gas exchange measurements, are often used to study water use.
Sap flow sensors are essential tools for studying how plants use water and how they adapt to environmental stresses. By measuring the movement of water through the xylem, sap flow sensors provide valuable insights into transpiration rates, plant water-use efficiency, and the impacts of factors like drought, heat, and soil moisture availability on plant water relations. These measurements are critical for improving agricultural water management, studying drought tolerance, and managing ecosystems in the face of climate change.
9. Stomatal Aperture Bioassays
Stomatal aperture bioassays are experimental techniques used to investigate the opening and closing of stomata, the small pores on the surfaces of leaves and stems that regulate gas exchange and water loss in plants. Stomatal movements are crucial for photosynthesis, respiration, and maintaining the plant’s water balance. Understanding the mechanisms that control stomatal aperture is vital for plant physiology research, especially in the context of environmental stresses such as drought and high temperatures.
The process of conducting stomatal aperture bioassays typically involves several key steps. First, researchers select appropriate plant species or varieties for the study, often opting for model organisms like Arabidopsis thaliana or economically significant crops such as rice, wheat, or soybean, as these plants have well-characterized stomatal behavior. Once the plant species is chosen, fresh leaf samples are collected. Leaves are usually excised early in the morning, when stomata are typically open, although they can be collected at various times to study specific conditions. To ensure consistency in measurements, researchers cut leaf discs or strips to a standardized size.
After leaf preparation, researchers apply various treatments to assess their effects on stomatal aperture. These treatments can include altering light intensity to see how different lighting conditions influence stomatal opening, adjusting humidity levels to assess their impact on stomatal conductance, applying plant hormones like abscisic acid (ABA) to study its role in inducing stomatal closure during drought, or exposing leaves to environmental stresses such as elevated temperatures, drought, or salinity.
Once the treatments are applied, researchers measure the stomatal apertures. This measurement can be conducted using several techniques. Microscopy, including light microscopy or scanning electron microscopy (SEM), allows for the visualization and measurement of stomatal size and opening. Digital image analysis software can also be used to quantify stomatal apertures from captured images, enabling more precise and automated measurements.
After measuring the stomatal apertures, data analysis involves comparing results from different treatments to determine significant differences in stomatal behavior under varying conditions. Statistical methods can be employed to assess the impact of treatments on stomatal movements, providing insights into how environmental factors influence these critical processes.
The applications of stomatal aperture bioassays are extensive in plant physiology and related fields. One primary application is to understand stomatal regulation. By studying how various environmental factors and plant hormones affect stomatal aperture, researchers can gain insights into the physiological mechanisms and signaling pathways involved in stomatal movements. These bioassays are also instrumental in examining how plants respond to abiotic stresses like drought and heat. Understanding the reactions of stomata under stress conditions is crucial for breeding programs aimed at developing more resilient crops that can withstand such challenges.
Additionally, stomatal bioassays contribute to photosynthesis research, as stomatal behavior directly impacts gas exchange and, consequently, photosynthesis. By analyzing stomatal movements, researchers can better understand the balance between water loss and carbon dioxide uptake, which is essential for improving crop productivity.
Furthermore, these bioassays can be utilized in ecophysiological studies to assess how different plant species adapt to their environments, particularly regarding water use efficiency and drought tolerance. Insights gained from stomatal aperture bioassays can also inform agricultural management practices. The information obtained can help optimize irrigation strategies or assist in selecting crop varieties that exhibit better water use efficiency in varying environmental conditions.
10. X-ray Microtomography
X-ray microtomography (XMT) is a sophisticated imaging technique that enables high-resolution, three-dimensional visualization of internal structures in a wide range of materials, including biological specimens, soil samples, and various plant tissues. This non-destructive method provides detailed insights into the architecture of complex systems without altering or damaging the samples, making it an invaluable tool in plant sciences, agriculture, and environmental research.
The fundamental principle of X-ray microtomography involves taking multiple X-ray images of a specimen from various angles. During the process, an X-ray source emits radiation, which passes through the sample and is detected by a sensor on the opposite side. Because different materials attenuate X-rays to varying degrees, the resulting images capture the density and composition of the sample. By rotating the sample and acquiring numerous two-dimensional (2D) projections, these images can be reconstructed into a comprehensive three-dimensional (3D) representation using advanced computational algorithms.
One of the key advantages of XMT is its ability to visualize structures at a micrometer resolution. This capability is particularly beneficial for studying plant anatomy, where researchers can examine the detailed organization of tissues, cells, and vascular systems. For example, X-ray microtomography can be used to analyze root architecture, providing insights into how roots interact with soil, explore nutrient uptake mechanisms, and respond to environmental stresses such as drought or salinity. This is essential for understanding plant adaptation and resilience in varying conditions.
XMT is also valuable for studying the internal structures of seeds and fruits. By visualizing the distribution of tissues and their respective densities, researchers can gain insights into seed development, dormancy mechanisms, and the effects of environmental factors on seed quality. In addition, XMT can help assess the microstructure of wood and other plant materials, which is important for applications in forestry and bioengineering.
In addition to biological applications, X-ray microtomography plays a significant role in environmental research. For instance, it can be employed to analyze soil structure and pore connectivity, providing vital information about water retention, root penetration, and nutrient transport within the soil matrix. This knowledge is essential for understanding plant-soil interactions and optimizing agricultural practices.
The technique also has applications in studying the effects of biotic and abiotic stresses on plant structures. Researchers can monitor changes in internal structures under stress conditions, such as drought or pathogen attacks, allowing them to elucidate the mechanisms of plant responses and adaptations. For example, XMT can reveal how stomatal conductance changes in response to environmental stress, thereby enhancing our understanding of water use efficiency in different plant species.
One of the notable strengths of X-ray microtomography is its non-invasive nature, allowing researchers to conduct longitudinal studies without altering the samples. This capability is particularly useful for studying developmental processes in plants, such as growth patterns and changes in tissue structure over time. By using XMT, researchers can visualize the same specimen at different stages of development, providing valuable insights into dynamic biological processes.
While XMT offers numerous advantages, there are some limitations to consider. The resolution of X-ray microtomography is often influenced by factors such as sample size, density, and the specific X-ray source used. Additionally, certain materials may require optimization of imaging parameters to achieve the best results, which can sometimes complicate the imaging process.
These techniques provide diverse approaches for understanding stomatal function and transpiration, offering insights into how plants cope with environmental stresses like heat and drought. Each method has its advantages depending on the research objectives and the environmental conditions being studied.
References
Jena, A., & Gupta, K. (2010). Advances in pore structure evaluation by porometry. Chemical engineering & technology, 33(8), 1241-1250.
Fowler, R. C. (1949). A rapid infra‐red gas analyzer. Review of Scientific Instruments, 20(3), 175-178.
Jones, H. G. (2004). Application of thermal imaging and infrared sensing in plant physiology and ecophysiology. In Advances in botanical research (Vol. 41, pp. 107-163). Academic Press.
Krause, G. H., & Weis, E. (1984). Chlorophyll fluorescence as a tool in plant physiology: II. Interpretation of fluorescence signals. Photosynthesis research, 5, 139-157.
Tyree, M. T., & Hammel, H. T. (1972). The measurement of the turgor pressure and the water relations of plants by the pressure-bomb technique. Journal of experimental Botany, 23(1), 267-282.
Long, S. P., & Bernacchi, C. J. (2003). Gas exchange measurements, what can they tell us about the underlying limitations to photosynthesis? Procedures and sources of error. Journal of experimental botany, 54(392), 2393-2401.
Timmers, A. C. (2016). Light microscopy of whole plant organs. Journal of microscopy, 263(2), 165-170.
Stabentheiner, E., Zankel, A., & Pölt, P. (2010). Environmental scanning electron microscopy (ESEM)—a versatile tool in studying plants. Protoplasma, 246, 89-99.
Baek, S., Jeon, E., Park, K. S., Yeo, K. H., & Lee, J. (2018). Monitoring of water transportation in plant stem with microneedle sap flow sensor. Journal of microelectromechanical systems, 27(3), 440-447.
Li, X., Ma, X. G., & He, J. M. (2013). Stomatal bioassay in Arabidopsis leaves. Bio-protocol, 3(19), e921-e921.
Karahara, I., Yamauchi, D., Uesugi, K., & Mineyuki, Y. (2015). Three-dimensional imaging of plant tissues using X-ray micro-computed tomography. Plant Morphology, 27(1), 21-26.